Brain-machine interfaces: How the Human Brain Shaped the Universe as We Know It
For people who are paralyzed, amputated, or suffering from motor-related neurological injury or disease, moving their hands or other parts of the body to accomplish an easy movement such as grabbing a cup of water can be extremely difficult. Their brains want to control their bodies, but for some reasons the control signals from brain are unable to delivery to their end effectors. Therefore, they can just think to execute the movement, but their bodies cannot execute their thoughts. In order to solve this problem, brain machine interfaces (BMIs) is developed. Brain Machine Interfaces (BMIs) is a technology to convert brain signals into commands to control a computer cursor or prosthetic devices. Ever since the first experimental demonstration in 1999 that ensembles of cortical neurons could directly control a robotic manipulator, there has been a significant progress in the basic research of BMIs study. To date, by implanting microarrays in the subject’s brain to record the neural signals, a intracortical BMI can successfully convert the neural signals into motor command to allow the paralyzed subject to control a robotic arm to grab a chocolate and eat it by her thoughts, or to stimulate the paralyzed muscles to achieve a desired movement.
The decoder was the most crucial component of a BMI; it modeled the functional mapping between neural activities and kinematic parameters (e.g. movement velocity or position), and assumed that this functional mapping was time-invariant (i.e., a stationary statistical assumption). However, within real neural recording conditions there existed a high degree of within- and across-day variability that prevented satisfaction of the stationary statistical assumption. This variability consisted of the relative position of the recording electrodes—and surrounding neurons, electrode properties, tissue reaction to electrodes, and neural plasticity—and might affect the functional mapping between neural activities and kinematic parameters. The variability in neural recording conditions resulted in unstable long-term decoding performance and led to frequent decoder retraining.
Conventional decoder retraining required the subject to periodically perform a well-defined task to collect new training data for preventing model staleness. This manner may lead to additional training time before the BMI can be used. Traditional linear neural decoders did not need frequent retraining but possessed limited computational complexity to deal with neural recording condition changes owning to linear properties. It has been known that the newly encountered neural recording conditions in chronic BMI systems have some commonality with past neural recording conditions. Therefore, computationally powerful nonlinear decoders were proposed to learn a diverse set of neural-to-kinematic mappings corresponding to various neural recording conditions collected in many days before BMI use. This manner avoided BMI interruption by keeping model parameters fixed during BMI use, and made BMI inherently robust to changes in neural recording conditions by exploiting the similarities between newly encountered and past neural recording conditions. Therefore, the BMIs were trained with numerous days of data in order to learn various neural recording conditions and achieve stable long-term decoding. However, they relied heavily on the huge training data where a large training set may not be available for some BMIs, e.g., rodent models. It is unknown whether the BMIs could deal with the scenario in which only a few days of training data were available.
We proposed an evolutionary constructive and pruning neural network with error feedback (ECPNN-EF) to decode neural activity into the forelimb movement of a rat by using only a few days of data to train the neural decoder. A lever-pressing task for the rat was designed to evaluate the effectiveness of the proposed neural decoder. The error feedback providing the difference between the decoded and actual kinematics might compensate for decreases in decoding performance when the neural recording conditions change. Thus, the ECPNN-EF might achieve stable and accurate long-term decoding performance. The rest of this paper is organized as follows. First, we describe the experimental setup and the proposed decoder. Second, we demonstrate the influence of several parameters, namely the probabilities of crossover and mutation. Furthermore, the effects of evolution progress, cluster-based pruning (CBP) and age-based survival selection (ABSS) on the performance of the proposed decoder are also shown and discussed. Finally, we describe how partially connected topology and error feedback improve the long-term decoding performance.

Fig2. The exoskelaton suit let's the user control movement through thought. Photo credit: The Walk Again Project.
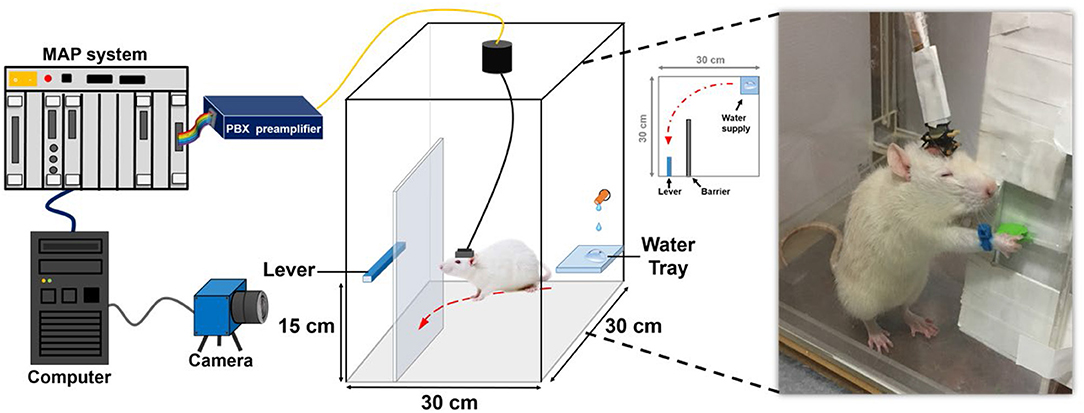
Fig3. System architecture and experimental setting of the water-reward lever-pressing task.
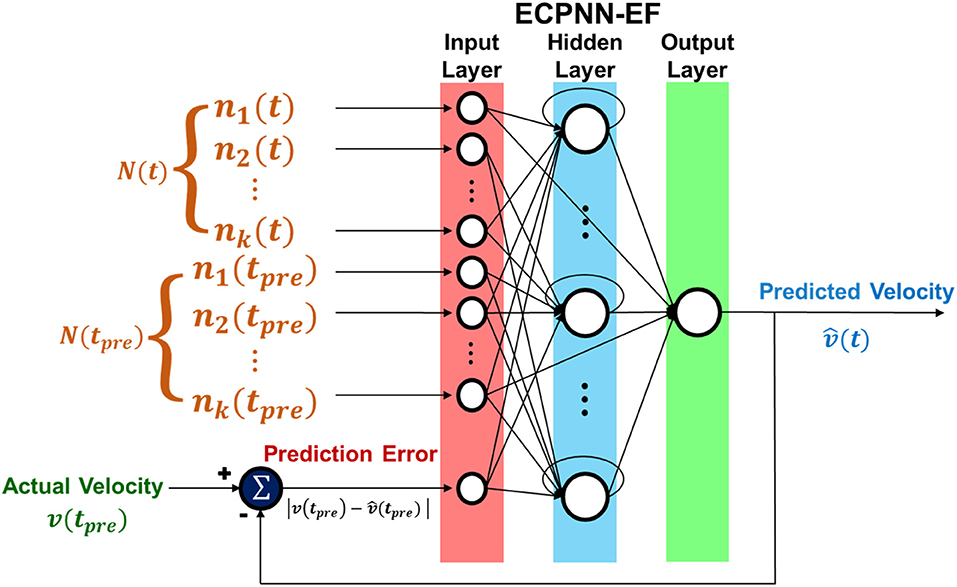
Fig4. Structure of ECPNN-EF
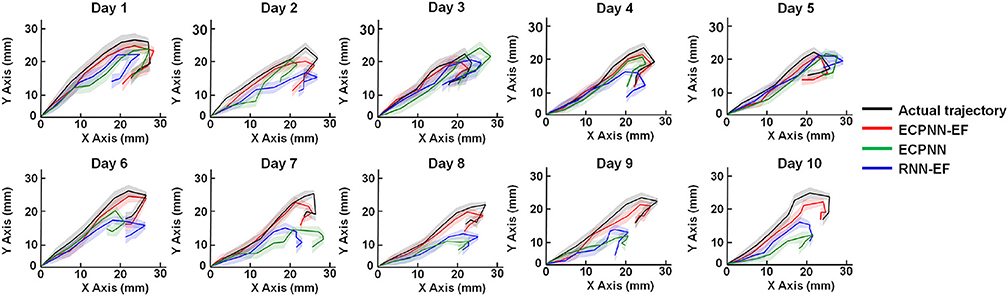
Fig5. Data visualization of average predicted trajectories of the ECPNN-EF, ECPNN, and RNN-EF.
Designed by: NTK Lab © Copyright 2020. All Rights Reserved.

